Bioanalytical research. Research in bio-analytical sciences (including microfluidics and lab-on-a-chip) is inherently interdisciplinary and requires expertise not only in biology, but also in engineering, physics, chemistry, mathematics, and computer science. Despite much progress in several universities, undergraduate students in engineering and the exact sciences have little, if any, exposure to biology. At the same time, biology students are traditionally not trained in quantitative techniques. Hence, few biology students transition into quantitative graduate work, and few quantitative sciences and engineering students are able to make the leap into the life sciences. Hence graduate students that choose to join a quantitative biology lab are typically woefully ill-prepared in at least one major aspect necessary for conducting high impact research projects in quantitative biology. As a result, the high promise and significant opportunities in this field remain largely unmet. We are hence highly interested in developing means of dissemination of our work as well involved in student training (in a small way!).
Progress in medicine and biology derives from observing and analyzing (complex) samples.
Microfluidics. Microfluidics leverages modern microtechnology to provide new tools for chemical and biological sciences. This field has benefited from silicon technology used to make microprocessors. The concept of “lab on a chip” seeks to replace traditional lab processes with effective alternatives implemented on a microchip. In additional to the direct gains from the use of very small sample and reagent volumes, many physical phenomena (e.g. diffusion, heat transfer, surface tension) scale favourably as size is reduced, allowing new capabilities and higher efficiencies in the analysis and control of samples.
Leveraging the unique microscale physics and chemistry to achieve new capabilities is at the core of microfluidics research.
Interestingly, while one of the tenants of lab on a chip field is miniaturization, which is primarily at the chip-level; there is often a disconnect between this form of miniaturization and that of the peripherals around it. Hence, much of instrumentation is used around the microfluidic chips, resulting in what is termed as “Chip in a lab”. This may or may not be problematic, depending on what is the objective of the project.
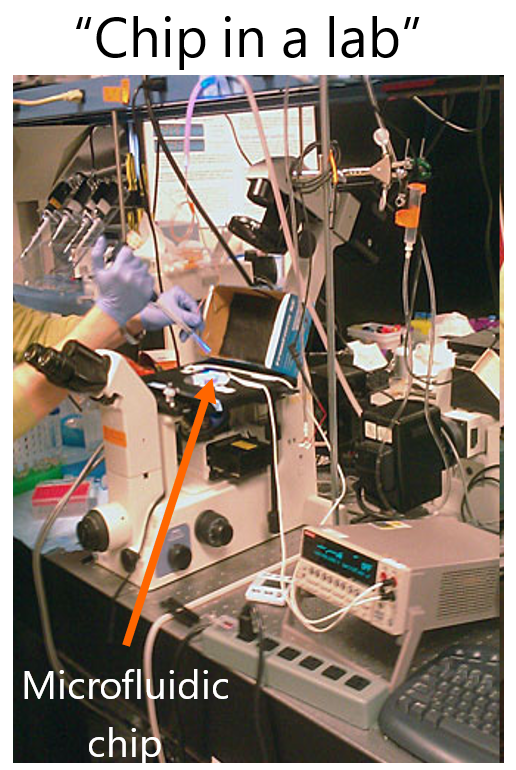
Closed (“conventional”) microfluidics. The vast majority of microfluidic devices currently uses networks of sealed microchannels (with four walls) and chambers that are linked to various inputs/outputs to provide a “chip-to-world” interface. In such devices, samples need to be either passed through, for example a biological sample potentially containing circulating tumor cells, or seeded in the device before sealing, for example by depositing adherent cells in microchambers.
Open space microfluidics. Open-space microfluidics provide new opportunities for handling, analyzing and interacting with biological samples and also give a lot of freedom to end-users for new class of experiments by removing the need for full containment of chemicals and liquids. This means reducing the number of walls in a microchannel that function to confine a liquid. This is done by creating well-defined chemical environments, typically around a specific area of a surface, using specific strategies for confining liquids. Certain open-space embodiments enable chemical reactions to be performed on immersed surfaces while completely eliminating gas–liquid interfaces. Alternatively, electrical forces can be used to direct charged species where desired on a surface.
Attributes of such a game changing technology:
-
Non-invasive: they should preferably work in a “non-contact” mode to minimize the perturbation of the biological interface.
-
Immersed: the presence of a buffer/liquid environment would prevent drying artifacts, such as denaturation.
-
Biocompatible: no toxic materials or chemicals should be needed, and the techniques should be compatible with typical ranges of pH values, temperature, ionic strengths, sheer forces, and pressures.
-
Flexible: compatibility with different materials, topographies, length scales (μm to cm), and volumes inherent to biological systems would be beneficial.
-
Interactive: the technique should provide feedback (e.g. current, voltage, force, optical signal) during interaction with the biological interface.
The microfluidic technology we are developing can control and build structured environments by altering the physics and chemistry of cells with cellular spatial granularity. Two examples of the technology we developed are:
Microfluidic probe (MFP). In line with the atomic force microscope and the scanning probe microscope developed at IBM-Zurich, the microfluidic probe (MFP) was conceived to process and interact with surfaces. The early versions of the MFP demonstrated suitability for working on biological surfaces. All liquid movement was performed either using syringe pumps or pressure-based actuation. A key step in the technology evolution was the transformation of the original format, which was essentially co-planar to scanned surfaces, into a vertical MFP technology. The vertically oriented MFP, now called the vMFP, is significantly expanding the versatility the probe. MFP permits patterning surfaces with proteins and other biomolecules in an additive and subtractive manner, forming complex gradients on surfaces, and interacting with cells on surfaces. With flow confinement operating at volumes smaller than 1 nanoliter, a few cells can be targeted in a human tissue section for the specific staining of disease markers. This confinement concept has also been scaled for targeting 1000’s of cells.
Eletrokinetic scanning probe (ESP). More recently, we presented a new concept for a non-contact scanning probe driven entirely by electric fields, which enables local interactions with confined reaction sites on biological and chemical interfaces in liquid environments. The electrokinetic actuation enables generation of stable, fluctuation-free and bubble-free flow confinements, which consume low volumes of samples, with essentially no dead volumes. The electrokinetic scanning probe (ESP) can accurately control the size of the confinement and the reaction conditions, and can be used for wet-in-wet printing, suitable for various biological applications. We demonstrate the compatibility of the ESP with a wide range of processing solutions, a broad range of pH and sample staking.
Key advantages of open space microfluidic technologies. Since cells & tissues will no longer be limited by closed systems, the systems such as the MFP or the ESP will enable a completely new range of experiments to be performed in a highly interactive, versatile & precise manner – this approach departs from classical “closed” microfluidics. It is very likely that such a tool by providing multifunctional capabilities akin to the proverbial ‘Swiss army knife’ will be a unique facilitator for investigations of previously unapproachable problems in cell biology & the life sciences.
For example, in the context of biopatterning, some new interesting regimes of operation could be obtained with hydrodynamic confinement-based patterning.
