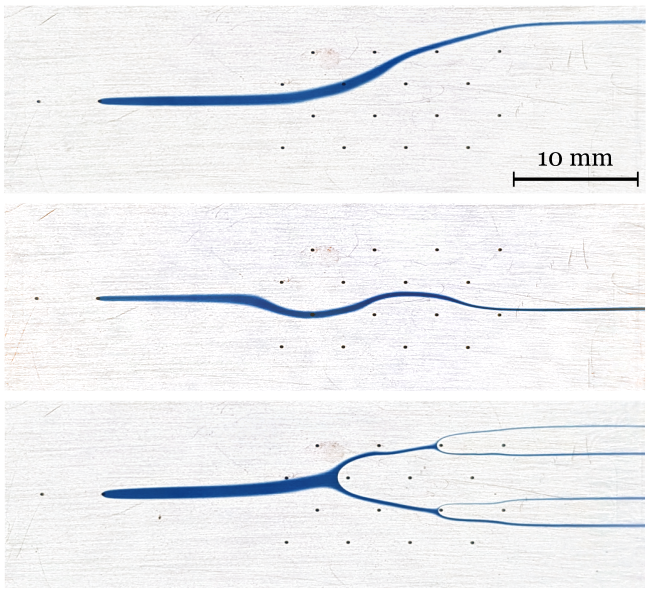
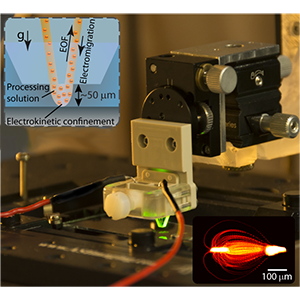
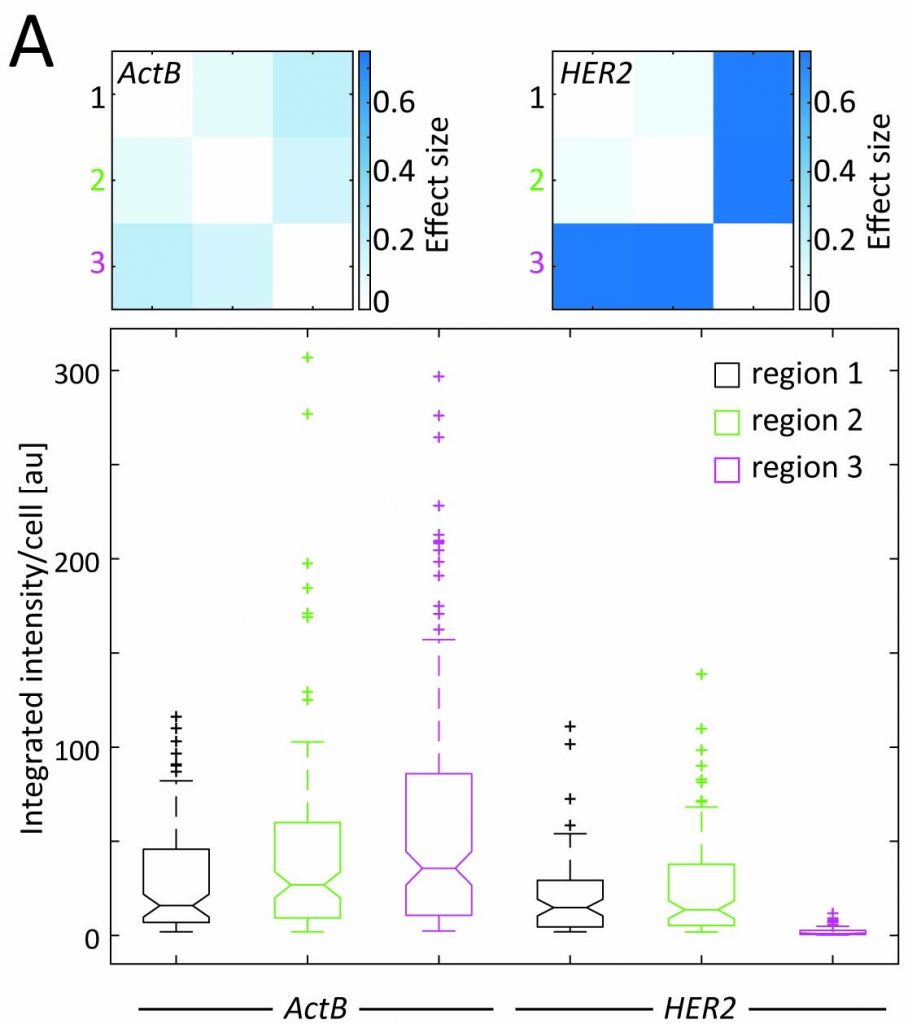
Featured Articles
Peer-Reviewed Publications
2022
[J.76] A. Fomitcheva-Khartchenko, A. Kashyap, T. Geiger, and G. V. Kaigala, Trends in Cancer, “Space in cancer biology: its role and implications,” 2022.
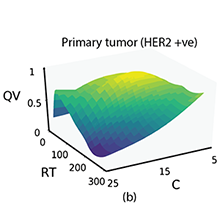
[J.75] P. Mathur, A. Fomitcheva Khartchenk, S. Stavrakis, G. V. Kaigala, and A. J. deMello, “Quantifying antibody binding kinetics on fixed cells and tissues via fluorescence lifetime imaging,” Analytical Chemistry, 2022 (Cover Page).
[J.74] D. P. Taylor, P. Mathur, P. Renaud and G. V. Kaigala, “Microscale hydrodynamic confinements: shaping liquids across length scales as a toolbox in life sciences” Lab Chip, 22, 1415-1437, 2022.
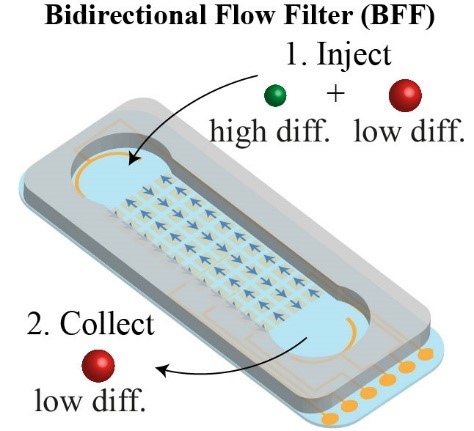
[J.73] V. Bacheva, F. Paratore, M. Bar-Dolev, B. Rofman, G. V. Kaigala, M. Bercovici, “Selective extraction of biomolecules using a bidirectional flow filter,” Analytical Chemistry, 2022.
[J.72] I. Pereiro, A. Fomitcheva Khartchenko, R. D. Lovchik, and G. V. Kaigala, “Simple add-on devices to enhance the efficacy of conventional surface immunoassays implemented on standard labware,” Analyst, 147, 2040-2047, 2022 (Cover Page).
[J.71] F. Paratore, V. Bacheva, and M. Bercovici, and G. V. Kaigala, “Reconfigurable microfluidics,” Nature Reviews Chemistry, 6, pages 70–80, 2022.
2021
[J.70] A. Kashyap, M. Rapsomaniki, A. Fomitcheva Khartchenko, V. Barros, A. L. Martinelli, A. Foncubierta Rodriguez, M. Gabrani, M. Rosen-Zvi, G.V. Kaigala, “Tumor heterogeneity: from sample to measurement to metric generation” Trends in Biotechnology, 2021.
[J.69] A. Fomitcheva Khartchenko, M. Rapsomaniki, B. Sobottka, P. Schraml, and G. V. Kaigala, “Local protein heterogeneity analysis in frozen tissues to evaluate tumor heterogeneity,” PLoS One, 2021.
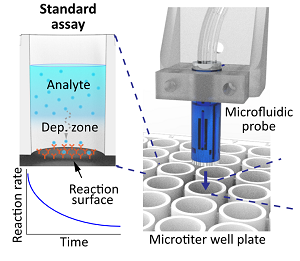
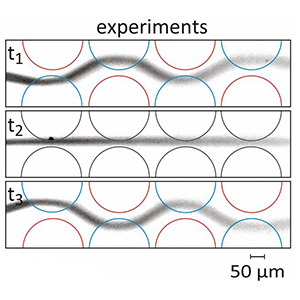
[J.68] I. Pereiro, A. Fomitcheva Khartchenko, R. D. Lovchik, and G. V. Kaigala, “Convection-enhanced kinetics in microtiter plates for improved surface assay quantitation and multiplexing capabilities,” Angewandte Chemie International Edition, p. 20935-20942, 2021.
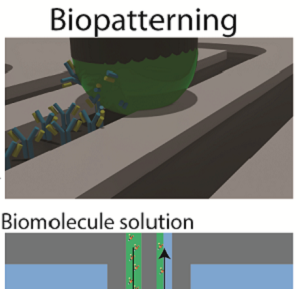
[J.67] E. Delamarche, I. Pereiro, A. Kashyap, G. V. Kaigala, “Biopatterning: The Art of Patterning Biomolecules on Surfaces,” Langmuir, 2021.
[J.66] I. Pereiro, A. Fomitcheva Khartchenko, R. Lovchik, G. V. Kaigala, “Advection-enhanced kinetics in microtiter plates for improved surface assay quantitation and multiplexing capabilities,” Angewandte Chemie International Edition, 2021.
[J.65] I. Pereiro, J. Aubert, and G. V. Kaigala, “Micro-scale technologies propel biology and medicine”, Biomicrofluidics 15(2), 2021.
[J.64] L. von Voithenberg, A. Kashyap, L. Opitz, C. Aquino, T. Sykes, M. Nieser, L.F.T. Petrini, N. E. Casimiro, X. F. van Kooten, S. Biskup, R. Schlapbach, P. Schraml, and G. V. Kaigala, “Mapping Spatial Genetic Landscapes in Tissue Sections through Microscale Integration of Sampling Methodology into Genomic Workflows”, Small, 2021.
[J.63] Widerker, D., Paratore, P., Bercovici, M., and Kaigala, G.V., “Biointegrated Fluidic Milling,” Advanced Materials Technologies 6(2), 2021.
2020
[J.62] R. D. Lovchik, D. Taylor, and G. V. Kaigala, “Rapid micro-immunohistochemistry“, Microsystems & Nanoengineering, 2020.
[J.61] P. Mathur, A. F. Khartchenko, A. deMello, and G. V. Kaigala, “Open space diffusive filter for simultaneous species retrieval and separation,” Analytical Chemistry, 2020.
[J.60] I. Pereiro, A. F. Khartchenko and G. V. Kaigala, “Shake it or flow it: Mass transport and kinetics in surface bioassays using agitation and microfluidics,” Analytical Chemistry, 2020.
[J.59] V. Bacheva, F. Paratore, S. Rubin, G. V. Kaigala and M. Bercovici, “Diffusion-based separation using bidirectional electroosmotic flow,”Angewandte Chemie International Edition, 2020.
[J.58] D. Taylor, and G. V. Kaigala, “Reconfigurable Microfluidics: Real-time Shaping of Virtual Channels through Hydrodynamic Forces,” Lab on a Chip, 2020.
[J.57] N. Ostromohov, B. Rofman, M. Bercovici and G. V. Kaigala, “Electrokinetic Scanning Probe“, Small, 2020.
2019
[J.56] L. V. Voithenberg, D. Huber, A. F. Khartchenko, P. Schraml, and G. V. Kaigala, “Spatially multiplexed RNA in situ hybridization to reveal tumor heterogeneity“, Nucleic Acids Research, 2019.
[J.55] X. van Kooten, L. F. T. Petrini, A. Kashyap, L. von Voithenberg, M. Bercovici, and G.V. Kaigala, “Spatially resolved genetic analysis of tissue sections enabled by microscale flow confinement retrieval and isotachophoretic purification” Angewandte Chemie International Edition, vol. 58, issue 43, 15259-15262, 2019. [blog]
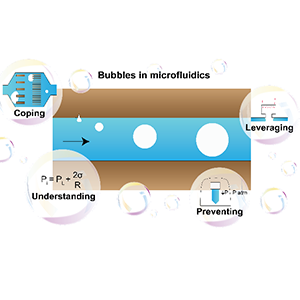
[J.54] I. Pereiro, A. Fomitcheva Khartchenko, L. Petrini, and G. V. Kaigala, “Nip the bubble in the bud: a guide to avoid gas nucleation in microfluidics,” Lab on a Chip, 19, 2296-2314, 2019. [popular press]
[J.53] F. Paratore F., E. Boyko, G. V. Kaigala, and M. Bercovici, “Electroosmotic Flow Dipole: Experimental Observation and Flow Field Patterning,” Physical Review Letters, 122, 224502, 2019.
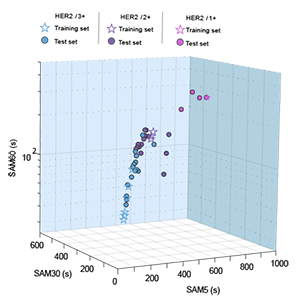
[J.52] A. Kashyap, A. Fomitcheva Khartchenko, P.Pati, M. Gabrani, P.Schraml, and G. V. Kaigala, “Quantitative microimmunohistochemistry for the grading of immunostains in tumor tissues,” Nature Biomedical Engineering, 3, 478–490, 2019. [blog 1] [blog 2] [blog 3]
[J.51] F. Paratore, V. Bacheva, G. V. Kaigala, and M. Bercovici, “Dynamic microscale flow patterning using electrical modulation of zeta potential” Proceedings of the National Academy of Sciences, 116 (21) 10258-10263, 2019. [blog] [PhysicsWorld]
[J.50] M. Arar, P. Pushpak, A Kashyap, A. Fomitcheva Khartchenko, O. Goksel, G. V. Kaigala, and M. Gabrani, “High-Quality Immunohistochemical Stains through Computational Assay Parameter Optimization, IEEE Transaction of Biomedical Engineering, 116 (21) 10258-10263, 2019.
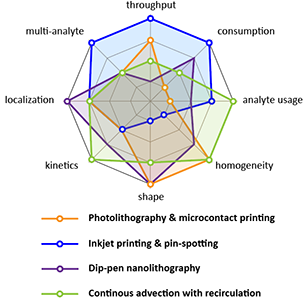
[J.49] I. Pereiro, J. F. Cors, S. Pané, B. J. Nelson, and G. V. Kaigala, “Underpinning transport phenomena for biopatterning” Chemical Society Reviews, 48, 1236-1254,2019 (Cover page). [blog]
2018
[J.48] D. P. Taylor, and G. V. Kaigala, “Fluidic bypass structures for improving the robustness of liquid scanning probes”, IEEE Transactions on Biomedical Engineering, vol. 66, 9, 2018.
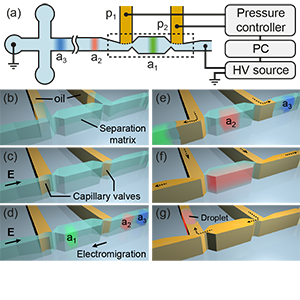
[J.47] X.F. van Kooten, M. Bercovici, and G.V. Kaigala, “Encapsulation-on-demand (EoD): a method for extraction of electrokinetically separated analytes in sub-nanoliter droplets,” Lab on a Chip,18(23), pp 3588-3597, 2018.
[J.46] D. Huber, L. von Voithenberg, and G.V. Kaigala, “Fluorescence in situ hybridization (FISH): history, limitations and what to expect from micro-scale FISH?” Micro and Nano Engineering,Vol 1, pp 15-24, Nov. 2018.
[J.45] N. Ostromohov, D. Huber, M. Bercovici and G.V. Kaigala, “Real-Time Monitoring of Fluorescence in situ Hybridization (FISH) Kinetics” Analytical Chemistry, 90 (19), pp 11470–11477, 2018 (Cover page). [blog]
[J.44] D. Huber, and G.V. Kaigala, “Rapid micro fluorescence in situ hybridization in tissue sections,”Biomicrofluidics, 12, 042212, 2018 (Cover page).
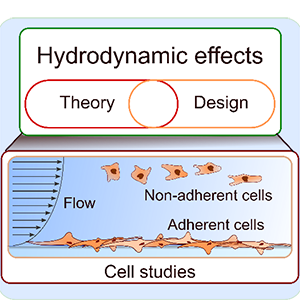
[J.43] D. Huber, A. Oskooei, X. C. Solvas, A. deMello, and G. V. Kaigala, “Hydrodynamics in cell studies,” Chemical Reviews, 118 (4), pp 2042–2079, 2018 (Cover page). [blog]
[J.42] A. Oskooei, and G. V. Kaigala, “Analyte-localization device for point-of-use processing of sub-millimetre areas on surfaces,” Sensors and Actuators – Chemical B, 258, 961-969, 2018.
2017
[J.41] X. F. van Kooten, M. Truman-Rosentsvit, G. V. Kaigala, M. Bercovici, “Focusing analytes from 50 μL into 500 pL: On-chip focusing from large sample volumes using isotachophoresis,” Scientific Reports, 7, Article number: 10467, 2017.
[J.40] F. Paratore, T. Zeidman Kalman, T. Rosenfeld, G. V. Kaigala, M. Bercovici, “Isotachophoresis-Based Surface Immunoassay” Analytical Chemistry, 89 (14), pp 7373–7381, 2017 (Cover page). [blog]
[J.39] J. F. Cors, A. Kashyap, A. Fomitcheva Khartchenko, P. Schraml, and G. V. Kaigala, “Tissue Lithography: Microscale Dewaxing to Enable Retrospective Studies on Formalin-Fixed Paraffin Embedded (FFPE) Tissue Sections,” PLoS One, May 11;12(5):e0176691, 2017.
[J.38] A. Oskooei, and G. V. Kaigala, “Deep-reaching hydrodynamic flow confinements (DR-HFC): µm-scale liquid localization for open surfaces with topographical variations, IEEE Transactions of Biomedical Engineering, 99, pp, 261 – 1269, 2017.
2016
[J.37] J. F. Cors, A. Stucki, and G. V. Kaigala, “Hydrodynamic thermal confinement: creating thermo-chemical microenvironments on surfaces,” Chemical Communications, 52, 13035–13038, 2016.
[J.36] D. P. Taylor, I. Zeaf, R. D. Lovchik, and G. V. Kaigala, “Centimeter-scale surface interactions using hydrodynamic flow confinements,” Langmuir, 32(41), 10537–10544, 2016.
[J.35] A. Kashyap, J. C. Cors, R. D. Lovchik, and G. V. Kaigala, “Rapid subtractive patterning of live cell layers with a microfluidic probe,” J. Vis. Exp. (115), e54447, 2016.
[J.34] A. Kashyap, J. Autebert, E. Delamarche, and G. V. Kaigala,”Selective local lysis and sampling of live cells for nucleic acid analysis using a microfluidic probe,” Scientific Reports, 6, 29579, 2016.
[J.33] N. Ostromohov, M. Bercovici, and G. V. Kaigala, “Delivery of minimally dispersed liquid interfaces for sequential surface chemistry,” Lab Chip, 16, 3015–3023, 2016. [special issue on emerging investigators] (Cover page).
[J.32] J. Autebert, J. Cors, D. Taylor, and G. V. Kaigala, “Convection-enhanced biopatterning with hydrodynamically confined nanoliter volumes of reagents,” Analytical Chemistry, 88(6), 3235–3242, 2016. (Cover page)
[J.31] D. Huber, J. Autebert, and G. V. Kaigala, “Micro fluorescence in situ hybridization (µFISH) for spatially multiplexed analysis of a cell monolayer,” Biomedical Microdevices, 18(40), 2016.
2015
[J.30] X. F. Kooten, J. Autebert and G.V. Kaigala, “Passive removal of immiscible spacers from segmented flows in a microfluidic probe,” Applied Physics Letters, 106, 074102, 2015.
[J.29] Y. Temiz, R.D. Lovchik, G.V. Kaigala and E. Delamarche, “Lab-on-a-chip devices” how to close and plug the lab?” Microelectronic Engineering, Volume 132, 25 January Pages 156-175, 2015.
2014
[J.28] M. Hitzbleck, G.V. Kaigala, E. Delamarche and R.D. Lovchik, “The floating Microfluidic Probe (fMFP): Distance control between probe and sample using hydrodynamic levitation,” Applied Physics Letters, 104, 263501, 2014.
[J.27] J. F. Cors, R. D. Lovchik, E. Delamarche and G.V. Kaigala, “Compact microfluidic probe,” Review of Scientific Instruments, 85, 034301, 2014.
[J.26] J. Autebert, A. Kashyap, R. D. Lovchik, E. Delamarche and G.V. Kaigala, “Hierarchical hydrodynamic flow confinement: efficient use and retrieval of chemicals for microscale biochemistry on surfaces,” Langmuir, Apr 1;30(12):3640-5, 2014.
2012
[J.25] G. V. Kaigala, R. D. Lovchik and E. Delamarche, “Microfluidics in the open space for local chemistries on biological interfaces,” Angewandte Chemie International Edition, Nov 5;51(45):11224-40, 2012.
[J.24] R. D. Lovchik, G. V. Kaigala, M. Georgiadis and E. Delamarche, “Micro-immunohistochemistry with the microfluidic probe,” Lab on a Chip, 12, 1040-1043, 2012 (Cover page).
[J.23] J. Jiang, G. V. Kaigala, H. J. Marquez and C. J. Backhouse, “Nonlinear controller designs for thermal management in PCR amplification,” IEEE Transactions on Control System Technologyogy, 11-30, 2012.
2011
[J.22] G. V. Kaigala, R. D. Lovchik, U. Drechsler and E. Delamarche, “A Vertical Microfluidic Probe,” Langmuir, 27, 5686-5693, 2011.
[J.21] M. Bercovici, G. V. Kaigala (co-first author), K. Mach, C. Han, J. Liao and J. G. Santiago, “Rapid detection of urinary tract infection using and isotachaphoresis and molecular beacons,” Analytical Chemistry, 83, 4110-4117, 2011.
[J.20] S. S. Bagha, G. V. Kaigala, M. Bercovici and J. G. Santiago, “High sensitivity indirect chemical detection using on-chip isotachophoresis with variable cross-section geometry,” Electrophoresis, 32, 563-572, 2011.
2010 and earlier
[J.19] G. V. Kaigala, M. Bercovici, M. Behnam, D. Elliott, J. G. Santiago and C. J. Backhouse, “Miniaturized system for isotachophoresis assays,” Lab on a Chip, 10, 2242-2250, 2010.
[J.18] M. Bercovici, G. V. Kaigala and J. G. Santiago, “Method for analyte identification using isotachophoresis and a fluorescent carrier ampholytes assay,” Analytical Chemistry, 82, 2134-2138, 2010.
[J.17] M. Bercovici, G. V. Kaigala, C. J. Backhouse and J. G. Santiago, “Fluorescent carrier ampholytes assay for portable, label-free detection of chemical toxins in tap water,” Analytical Chemistry, 82, 1858-1866, 2010.
[J.16] G. V. Kaigala, M. Behnam, A. C. E. Bidulock, C. Bargen, R. W Johnstone, D. G. Elliott and C. J. Backhouse, “A scalable and modular lab-on-a-chip genetic analysis instrument,” The Analyst, 1606-1617, 2010 (Cover page).
[J.15] M. Behnam, G. V. Kaigala (co-first author), M. Khorasani, S. Martel, D. G. Elliott and C. J. Backhouse, “Integrated circuit-based instrumentation for microchip capillary electrophoresis,” IET Nanobiotechnology, 4, 91-101, 2010.
[J.14] G. V. Kaigala, J. Jiang, C. J. Backhouse, H. J. Marquez, “System design and modeling of a time-varying nonlinear temperature controller for microfluidics,” IEEE Transactions on Control System Technology, 18, 521-530, 2010.
[J.13] G. V. Kaigala, M. Behnam, C. Bliss, M. Khorasani, S. Ho, D. G. Elliott, J. McMullin, and C. J. Backhouse, “Inexpensive, universal serial bus-powered and fully portable lab-on-a-chip-based capillary electrophoresis instrument,” IET Nanobiotechnology, 3,1-7, 2009.
[J.12] V. N. Hoang, G. V. Kaigala, A. Atrazhev, L. M. Pilarski, C. J. Backhouse, “Strategies for enhancing the speed and integration of microchip genetic amplification,” Electrophoresis, 29, 4684-4694, 2008 (Special issue DNA Sequencing).
[J.11] Y. Godwal, G. V. Kaigala (co-first author), V. Hoang, S-L. Lui, C. Backhouse, Y. Tsui, R. Fedosejevs, “Elemental analysis using micro Laser-induced Breakdown Spectroscopy (micro-LIBS) in a microfluidic platform,” Optics Express, 16, 12435-12445, 2008 (selected for publication to the Virtual Journal of Biomedical Optics).
[J.10] M. Behnam, G. V. Kaigala, M. Khorasani, P. Marshall, C. J. Backhouse, D. G. Elliott, “An integrated CMOS high voltage supply for lab-on-a-chip systems,” Lab on a Chip, 8, 1524–1529, 2008.
[J.9] G. V. Kaigala, V. Hoang and C. J. Backhouse, “Electrically controlled microvalves to integrate microchip polymerase chain reaction and capillary electrophoresis,” Lab on a Chip, 8, 1071-1078, 2008 (Cover page).
[J.8] W. H. Song, J. Kwan, G. V. Kaigala, V. N. Hoang and C. J. Backhouse, “Readily integrated, electrically-addressable microvalves,” Journal of Micromechanics and Microengineering, 18, 045009, 2008.
[J.7] G. V. Kaigala, V. Hoang, A. Stickel, J. Lauzon, D. Manage, L. M. Pilarski, C. J. Backhouse, “An inexpensive and portable microchip-based platform for integrated RT-PCR and capillary electrophoresis,” The Analyst, 133, 331–338, 2008 (Cover page, one of the three most accessed articles published in the journal Analyst for the year 2008).
[J.6] V. Hoang, G. V. Kaigala, C. J. Backhouse, “Dynamic temperature measurement in microfluidic devices using thermochromic liquid crystals,” Lab on a Chip, 8, 484-487, 2008.
[J.5] J. Chowdhury, G. V. Kaigala (co-first author), S. Pushpakom, J. Lauzon, A. Makin, A. Atrazhev, A. Stickel, W. G. Newman, C.J. Backhouse and L.M. Pilarski, “Microfluidic platform for Single Nucleotide Polymorphism genotyping of the Thiopurine S-Methyltransferase gene to evaluate risk for adverse drug events,” J. Molecular Diagnostics, 9, 521-529, 2007.
[J.4] J. VanDijken, G. V. Kaigala (co-first author), J. Lauzon, A. Atrazhev, B. J. Taylor, T. Reiman, A. R. Belch, C J. Backhouse, L. M Pilarski, “Microfluidic chips for detecting the t(4;14) translocation and monitoring disease during treatment using Reverse Transcriptase-Polymerase Chain Reaction analysis of IgH-MMSET hybrid transcripts,” J. Molecular Diagnostics, 9, 358-367, 2007.
[J.3] V. J. Sieben, C. S. Debes-Marun, P. M. Pilarski, G. V. Kaigala, L. M. Pilarski, C. J. Backhouse, “FISH and chips: Chromosomal analysis on microfluidic platforms,” IET Nanobiotechnology, 1, 25-37, 2007.
[J.2] G. V. Kaigala, S. Ho, R. Penterman, C. J. Backhouse, “Rapid prototyping of microfluidic devices with a wax printer,” Lab on a Chip, 7, 384-387, 2007.
[J.1] G. V. Kaigala, R. Huskins, J. Preiksaitas, X. Pang, L. M. Pilarski, C. J. Backhouse, “Automated screening using microfluidic chip-based PCR and product detection to assess risk of BK virus-associated nephropathy in renal transplant recipients,” Electrophoresis, 27, 3753, 2006.
Books and book chapters
Open-Space Microfluidics, Wiley 2018.
ISBN: 978-3-527-34038-5.
| Forward | Preface |
Total 18 chapters (listed below chapters contributed by G.V. Kaigala):

E. Delamarche, R. D. Lovchik, J.F. Cors and G.V. Kaigala,
Hydrodynamic Flow Confinement Using a Microfluidic Probe
J.F. Cors, J. Autebert, A. Kashyap, D.P. Taylor,
R.D. Lovchik, E. Delamarche, and G.V. Kaigala
Hierarchical Hydrodynamic Flow Confinement and Recirculation for performing Microscale Chemistry on Surfaces
R.D. Lovchik, D.P. Taylor, E. Delamarche, and G.V. Kaigala
Hydrodynamic flow confinement-assisted immunohistochemistry from micrometer to millimeter-scale
A. Kashyap, D. Huber, J. Autebert, and G.V. Kaigala
Local nucleic acid analysis of adherent cells
PhD thesis
[for each of the PhD dissertations, a professor from the university serves as the academic advisor and often also serves as a co-advisor]
David Taylor PhD, EPFL 2019
Thesis title: Microscale physico-chemical interactions between hydrodynamically confined liquids and impressed biological surfaces
Federico Paradore PhD, Technion 2019
Thesis title: Coupling eletrokinetics with patterned surfaces: from enhanced immunoassays to microscale flow control
Xander van Kooten PhD, Technion 2018
Thesis title: On-chip separation and focusing using isotachophoresis with large-volume processing and analyte encapsulation
Aditya Kashyap PhD, ETH-Z 2018
Thesis title: Tumor tissue and cancer cell microprocesing for local molecular analysis using microfluidic probe technology
Deborah Huber PhD. ETH-Z 2018
Thesis title: micro-scale fluorescence in situ hybridization
Julien Cors PhD, ETH-Z 2017
Thesis title: Microscale surface-based bioanalytical applications using microfluidic probe technologies
Masters thesis
[for each of the master’s thesis listed below, a professor from the university served as an academic supervisor, along with a a post-doc or a senior PhD student within the team co-supervise the masters thesis]
Lorenzo Mattolini M.Sc., EPFL 2019
Thesis title: Local mRNA analysis to investigate intra-tumor transcriptional heterogeneity in breast cancer
Bruno Charlety M.Sc., EPFL 2019
Thesis title: Pick-and-place of adherent cells with shear-stress and chemically controlled microenvironments created using a microfluidic probe
Vesna Bacheva M.Sc., EPFL 2018
Thesis title: Novel diffusion-based separation method
Nadia Enrriquez Casimiro M.Sc., University of Basel 2018
Thesis title: Study of cellular responses at the microscale by creating heterogeneity in cultured cells using a microfluidic probe
Lorenzo Petrini M.Sc., EPFL 2018
Thesis title: On-chip eletrokinetic pre-concentration and amplification of nucleic acids sampled locally on tissue sections.
Gema Vera Gonzalez M.Sc., ETH-Z 2017
Thesis title: Study of the cytotoxic effects of drugs on live cells using a microfluidic platform
Anna Khartchenko M.Sc., ETH-Z 2016
Thesis title: Quantitative micro-immunnohistochemistry using the microfluidic probe
Ismeal Zeaf M.Sc. EPFL 2015
Thesis title: Large area immunohistochemistry with hydrodynamically confined flows microfluidic probe.
Ariane Stucki Sophie M.Sc., EPFL 2015
Thesis title: Strategies for local heating of hydrodynamic confined liquids on surfaces with the microfluidic probe
Radostina Eneva M.Sc. ETH-Z 2015
Thesis title: Localized fluorescence in situ hybridization with a microfluidic probe
Xander van Kooten M.Sc., EPFL 2014
Thesis title: Strategies for rapid switching of liquids in hydrodynamically confined flows
Aditya Kashyap M.Sc., ETH-Z 2014
Thesis title: Local DNA extraction from tissue sections using the microfluidic probe
Julien Cors M.Sc., ETH-Z 2013
Thesis title: Compact microfluidic probe
Marios Georgiadis M.Sc., ETH-Z 2011
Thesis title: Microfluidic probe for local staining of tissue sections